how to reduce aldehyde to alcohol Nabh4 aldehydes alcohols different
Alcohol, aldehyde, and carboxylic - three different organic compounds that have different properties and different reactions. These compounds react differently when exposed to oxidizing agents, which can lead to the formation of different chemical products. In this post, we will discuss the process of oxidation of these compounds and the chemical products formed.
Oxidation Process of Alcohol, Aldehyde, and Carboxylic
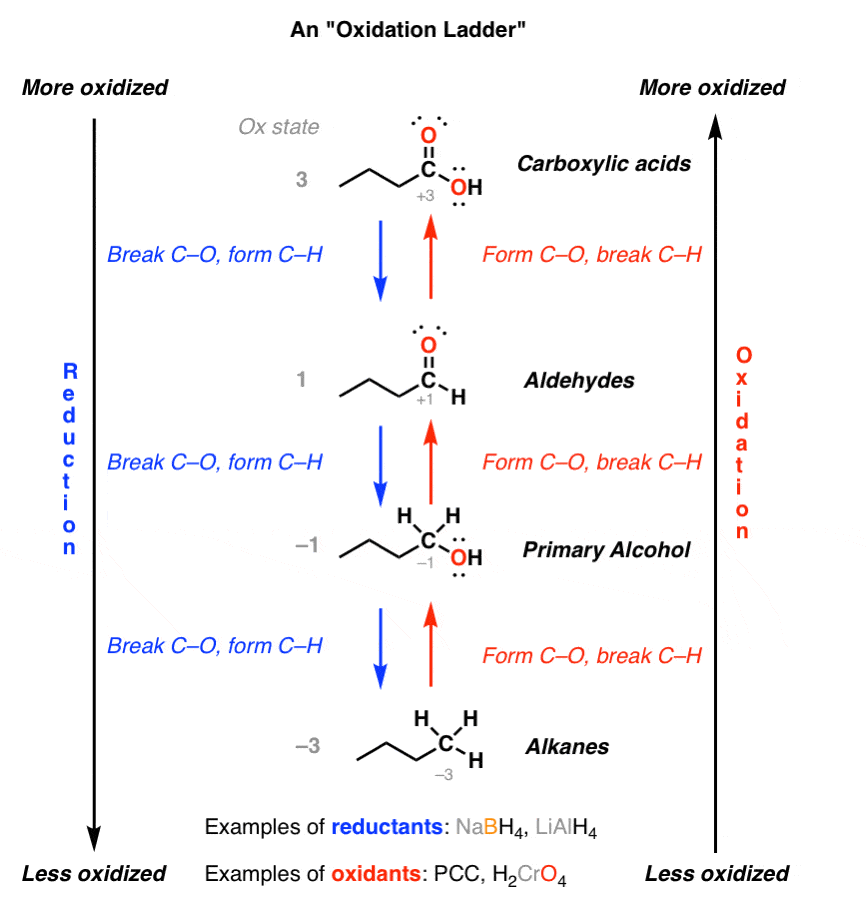 The oxidation of alcohol, aldehyde, and carboxylic is an essential process in organic chemistry. This reaction involves the removal of two hydrogen atoms from the organic compound and replacing them with an oxygen atom.
The oxidation of alcohol, aldehyde, and carboxylic is an essential process in organic chemistry. This reaction involves the removal of two hydrogen atoms from the organic compound and replacing them with an oxygen atom.
Alcohol is the primary organic compound that undergoes the oxidation process. It is typically oxidized using strong oxidizing agents, such as potassium permanganate or chromic acid. During the oxidation process, a primary alcohol is converted to an aldehyde and then to a carboxylic acid.
An aldehyde can also be oxidized using mild oxidizing agents, such as Tollens’ reagent or Fehling’s solution. Aldehydes are converted to carboxylic acids during the oxidation process.
Lastly, a carboxylic acid can also undergo oxidation to form a carbon dioxide molecule and water. This process is typically performed using a mild oxidizing agent, such as a hydrogen peroxide solution.
Ketone and Aldehyde Reduction by NaBH4
 NaBH4 is a popular reducing agent used in organic chemistry to reduce ketones and aldehydes. The mechanism of reduction involves the transfer of a hydride ion (H-) from NaBH4 to the carbonyl group of the aldehyde or ketone. The result of which is a reduction of the carbonyl group to an alcohol.
NaBH4 is a popular reducing agent used in organic chemistry to reduce ketones and aldehydes. The mechanism of reduction involves the transfer of a hydride ion (H-) from NaBH4 to the carbonyl group of the aldehyde or ketone. The result of which is a reduction of the carbonyl group to an alcohol.
The mechanism of reduction by NaBH4 is a two-step process. The first step involves the nucleophilic attack of the hydride ion on the carbonyl carbon atom. The second step involves the protonation of the oxygen atom, which creates an alcohol molecule.
The reduction of aldehydes by NaBH4 is faster than ketones because aldehydes have a carbonyl group that is more electrophilic than ketones. This means that the carbonyl group of aldehydes is more susceptible to nucleophilic attack by the hydride ion in NaBH4.
In conclusion, oxidation and reduction reactions are essential processes in organic chemistry. The oxidation process of alcohol, aldehydes and carboxylic provides a pathway to various products, including carboxylic acid from alcohol and carbon dioxide from carboxylic acid. NaBH4 is an effective reducing agent capable of reducing ketones and aldehydes to form an alcohol molecule. The mechanisms of these reactions are interesting and show how different compounds react differently.
If you are looking for Addition of NaBH4 to aldehydes to give primary alcohols – Master you’ve came to the right place. We have 5 Pictures about Addition of NaBH4 to aldehydes to give primary alcohols – Master like Addition of NaBH4 to aldehydes to give primary alcohols – Master, New Page 2 [www.chemhume.co.uk] and also Flow chart of oxidation process of alcohol ,aldehyde and carboxylic. Here you go:
Addition Of NaBH4 To Aldehydes To Give Primary Alcohols – Master
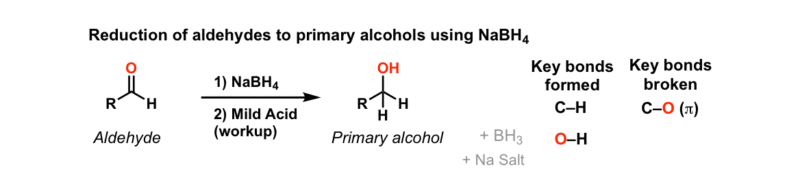 www.masterorganicchemistry.comnabh4 aldehydes alcohols reduction
www.masterorganicchemistry.comnabh4 aldehydes alcohols reduction
New Page 2 [www.chemhume.co.uk]
![New Page 2 [www.chemhume.co.uk]](http://chemhume.co.uk/A2CHEM/Unit 1/3 Carbonyl groups/reduction_of_aldehyde.jpg) www.chemhume.co.ukreduction aldehyde aldehydes carbonyl
The Mechanism Of Ketone And Aldehyde Reduction By NaBH4 | Organic
 www.pinterest.clreduction mechanism nabh4 chemistry ketone organic aldehyde sodium carbonyl borohydride lialh4 ketones alcohol carboxylic ester aldehydes group esters nabh between
www.pinterest.clreduction mechanism nabh4 chemistry ketone organic aldehyde sodium carbonyl borohydride lialh4 ketones alcohol carboxylic ester aldehydes group esters nabh between
Flow Chart Of Oxidation Process Of Alcohol ,aldehyde And Carboxylic
 www.meritnation.comoxidation aldehyde carboxylic plzzzz
www.meritnation.comoxidation aldehyde carboxylic plzzzz
Addition Of NaBH4 To Aldehydes To Give Primary Alcohols – Master
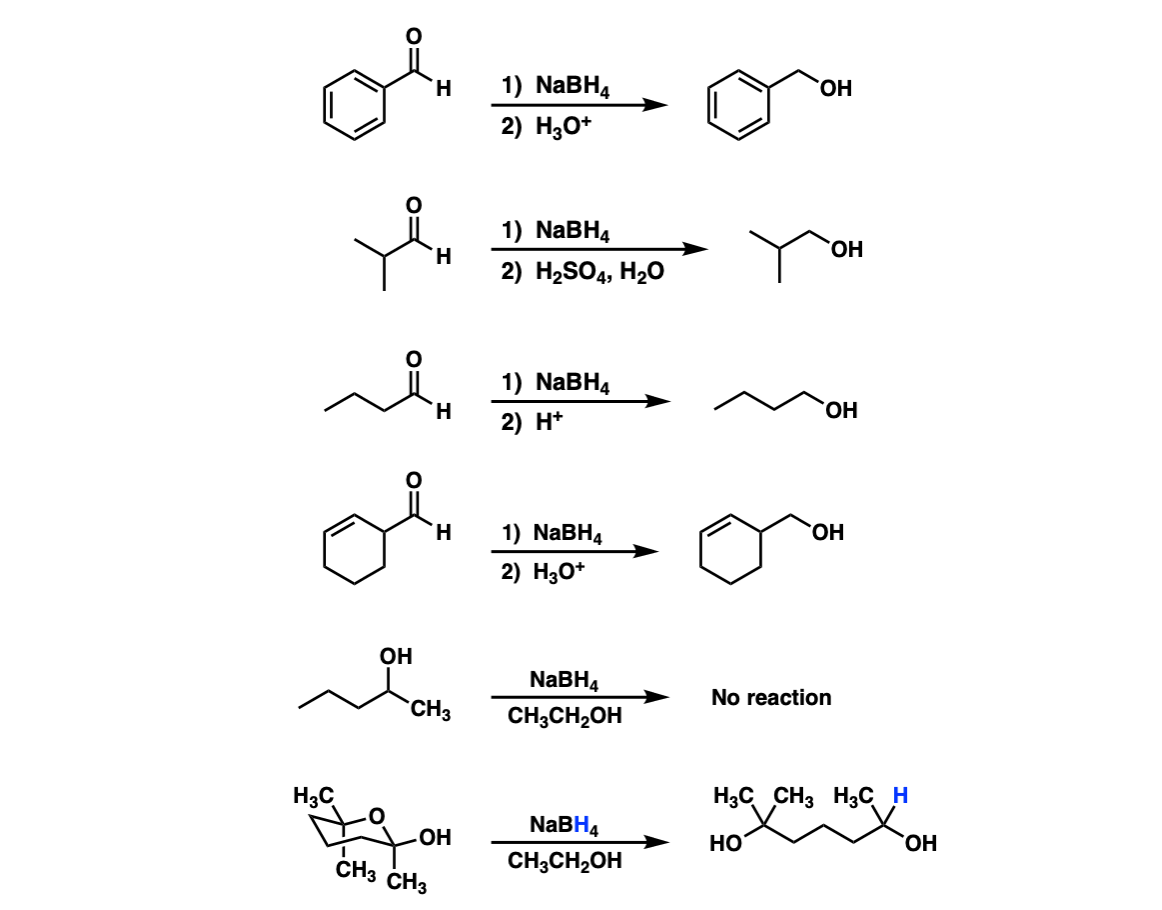 www.masterorganicchemistry.comnabh4 aldehydes alcohols different
www.masterorganicchemistry.comnabh4 aldehydes alcohols different
Reduction aldehyde aldehydes carbonyl. Nabh4 aldehydes alcohols reduction. Addition of nabh4 to aldehydes to give primary alcohols – master